In today’s highly regulated global environment, pharmaceutical, biotech and medical device companies are under increasing pressure to demonstrate compliance across their extended third-party networks. From anti-bribery enforcement to FDA debarment risk to global supply chain integrity, life sciences organizations need more than just spreadsheets and checklists to manage their third-party risk.
A modern SaaS-based third-party due diligence platform is no longer a luxury — it’s a regulatory and operational imperative.
Why Third-Party Risk Management Is Critical in Life Sciences
Financial, data privacy, and cybersecurity risks to the business posed by vendors, subcontractors, distributors and service providers span multiple domains:
- Compliance and Regulatory Risk: Engagements with debarred or sanctioned entities can trigger FDA, OIG/DOJ, SEC, and even OFAC enforcement actions or ABAC/FCPA violations.
- Operational Risk: A Contract Resource Organization (CRO) with quality system deficiencies can jeopardize an entire clinical program.
- Reputational Risk: Third-party misconduct (e.g., bribery, data mishandling) can harm brand credibility and trigger shareholder scrutiny.
Given the complexity of global operations and the scrutiny from regulators, a fragmented approach to due diligence simply won’t cut it.
Essential Capabilities of a SaaS-Based Third-Party Due Diligence Platform
1.) Sanctions, Debarment and Watchlist Screening
Screening third parties, their principals, and other key stakeholder relationships against government and international lists is mandatory. This includes:
- OFAC and EU Sanctions Lists
- United Nations Security Council (UNSC)
- FDA Debarment List
- OIG LEIE (List of Excluded Individuals/Entities)
- SAM.gov exclusions
- Interpol Red Notices
- World Bank Debarment
- Politically Exposed Persons (PEP) Lists
- US Federal and State Exclusion Databases
Although the above may be mandatory, conducting a more thorough check against state-owned entities (SOEs) and state-invested entities (SIEs) has been overlooked. Given the geopolitical environment, this is a must.
Advanced platforms such as the solution we offer at MediSpend, also monitor politically exposed persons (PEPs) and perform adverse media screening, identifying potential risk signals beyond formal databases.
2.) Customizable Risk Assessments & Due Diligence Questionnaires
Different third-parties present different levels of risk. Look for platforms that support:
- Tiered, dynamic questionnaires
- Triggers for enhanced due diligence based on geography, business activity or spend level
- Configurable workflows for internal approvals and escalations
3.) Third Party Portal for Engagement & Certification
A self-service vendor portal significantly reduces administrative burden and improves audit readiness. Key portal functions should include:
- Questionnaire and document submission
- Annual recertification
- Policy acknowledgment tracking
- Real-time status updates and two-way communication
Ensure that your current solution enables the ability to create custom questionnaires and has the capability of selecting from a library of assessments.
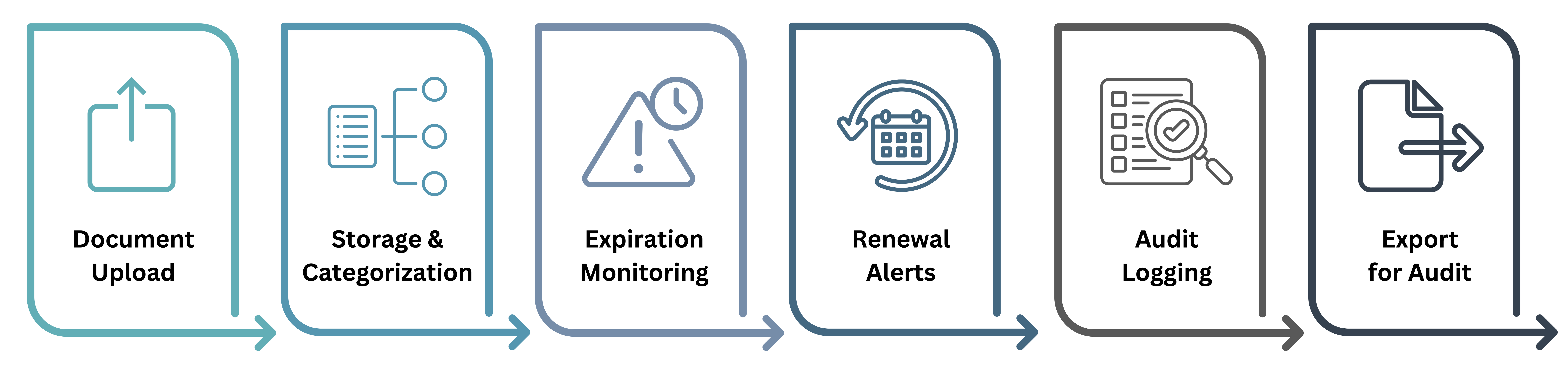
4.) Document Management and Audit Trails
Maintaining a central, searchable record of vendor certifications, agreements and due diligence artifacts is crucial. Features should include:
- Secure upload and storage of contracts, licenses, NDAs, QMS certifications
- Expiration date tracking and renewal alerts
- Full audit logs and exportable histories for regulatory audits
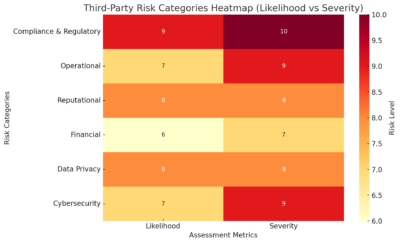
5.) Risk Scoring & Dashboards
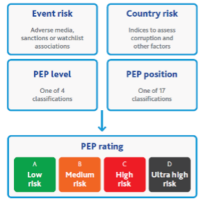 Compliance and procurement teams require clear visibility to effectively identify and address all risks. A modern platform should:
Compliance and procurement teams require clear visibility to effectively identify and address all risks. A modern platform should:
- Score vendors based on questionnaire responses, screening results and risk logic
- Display real-time dashboards with heat maps, KPIs and risk summaries
- Integrate with BI tools or export for reporting, or better yet, offer an out-of-box BI tool
Any provider of a third-party due diligence platform should already offer business intelligence capabilities that enable your organization to proactively monitor risk through dashboard and ad-hoc reporting capabilities that are configured based on your company policies and procedures.
6.) Continuous Monitoring
Risk doesn’t stop after onboarding. Leading platforms provide:
- Automated ongoing screening for sanctions, legal issues or media hits
- Alerts on corporate ownership changes or executive turnover
- Reassessment triggers based on predefined thresholds
7.) Optional: Contract Lifecycle Management (CLM)
While not required, having contracting tools enhances governance, especially for high-risk vendors. Some platforms allow:
- Pre-approved templates for NDAs, MSAs and quality agreements
- E-signature integrations (e.g., DocuSign)
- Clause-level version tracking and legal review workflows
Best-in-class third-party due diligence solutions come with an end-to-end contracting capability because you shouldn’t have to leverage multiple different systems to unify your company’s third-party due diligence program.
Conclusion: Don’t Let Risk Hide in Plain Sight
The cost of failing to manage third-party risk is high — not just in fines and disruptions, but in trust, market share and patient impact.
Life sciences companies need a platform that goes beyond basic checklists to deliver real-time insight, regulatory alignment and operational efficiency. A well-designed SaaS platform helps compliance, legal, procurement and risk teams work together to prevent problems before they start via a cost-effective approach.
Don’t put your business at risk! MediSpend’s third-party due diligence solution will provide your organization with peace of mind while protecting your bottom line and your reputation.
Ready to modernize your third-party due diligence process? Request a demo today.